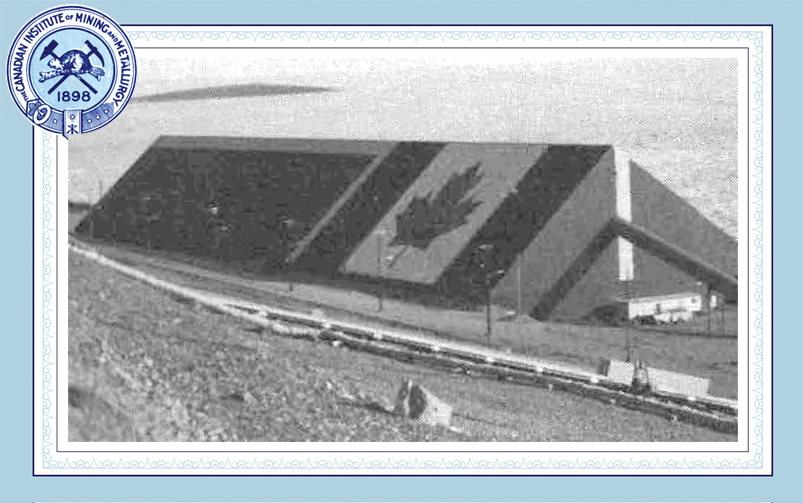
Neil Colvin operating a Sherritt pilot plant autoclave in Ottawa circa 1951 (CIM Journal, 2018)
The Sherritt Gordon ammonia leach process was originally developed to process nickel-copper sulfide ore from deposits in Lynn Lake, Manitoba.
“It was [the] fortunate turn of events that brought Sherritt Gordon and Chemico together, and the piloting that followed, that gave birth to commercial pressure hydrometallurgy,” wrote M.E. Chalkley, P. Cordingley, G. Freeman, J. Budac, R. Krentz and H. Scheie (Proceedings of Pressure Hydrometallurgy 2004).
Sherritt Gordon Mines had been operating a copper-zinc mine at Sherridon, Manitoba, since 1931, but by 1946 it was estimated that it had just four years of production left. The company intended to use the mining and milling equipment from Sherridon to treat ore from a planned mine at Lynn Lake, approximately 240 kilometres to the north.
Professor Frank Forward, then head of the department of mining and metallurgy at the University of British Columbia (UBC), wrote in CIM Bulletin (November 1953) that “a number of unusual problems were faced in transferring plant and operations from the one mine to the other, and particularly in preparing to undertake nickel production.”
Preliminary tests of the Lynn Lake ore at Sherritt’s laboratory in Sherridon showed that it could be concentrated by flotation to produce a copper concentrate that contained 28 to 30 per cent copper with less than one per cent nickel, as well as a nickel concentrate containing 11 to 14 per cent nickel, 0.3 to 0.4 per cent cobalt and less than two per cent copper, with high recovery of the three metals in the concentrates, according to Forward (1953).
However, the economics were unattractive for a standalone smelter at Lynn Lake. “It was indicated that operating costs might be high due to the location of the mine, the cost and availability of fuel, fluxes and power, and the high labour requirement usually associated with smelting and refining processes,” wrote Forward (1953). “Another disadvantage of these processes is that much of the cobalt is usually lost in the smelting operation.”
Research and development
The results of this early work were promising enough to convince Sherritt Gordon to finance a test program. The company sent Forward some nickel concentrate samples from Lynn Lake for evaluation.
“Although the period between discovery and commercial operation of a new process is usually about 10 years, the development period had to be considerably shorter than this, as it was necessary to start production at Lynn Lake as soon as possible after the Sherridon mine was shut down,” wrote Forward (1953). “This meant that the maximum period available was about five years.”
In 1947 and early 1948, the Sherritt-funded test program at UBC focused on adapting the Caron process—recently commercialized at Nicaro in Cuba to treat nickel laterite ore—to roasted Lynn Lake concentrate.
“With the introduction of certain variations, [it] could be used to extract and recover both nickel and copper from a nickel-copper sulfide concentrate, ore or matte,” wrote F.A. Forward, C.S. Samis and V. Kudryk (CIM Bulletin, June 1948). “If electrolytic hydrogen could be employed as a reducing agent, the process might be operated in an area where low-cost power is available.”
However, D.G.E. Kerfoot (CIM Bulletin, June 1999) noted that during the course of their work, Forward’s team stumbled on the discovery that nickel and copper sulfides could be leached directly in the ammoniacal ammonium carbonate solution in an oxygen atmosphere.
Sherritt Gordon, Forward and Chemical Construction Corporation (Chemico)—a subsidiary of American Cyanamid that had also been studying techniques for pressure leaching sulfide ores—met in December 1948 to discuss the design and engineering of a pilot plant to develop the novel ammonia leach process.
“Of particular interest was the fact that Chemico engineers had found that nickel could be readily precipitated from ammoniacal solutions by hydrogen under pressure. As it appeared that this technique would greatly simplify the production of nickel metal from the leach solution, plans were made to incorporate the hydrogen reduction treatment in the ammonia leach process,” wrote Forward (1953).
Sherritt and Chemico collaborated on a series of four increasingly sophisticated pilot plants, through which the commercial process was developed.
“The comprehensive investigations carried out in laboratories and pilot plant during the next four years provided extensive technical information on a process in which the nickel sulfide concentrate is leached with ammonia and compressed air, the nickel, copper and cobalt are dissolved and subsequently separated and recovered, and the sulfur is converted to ammonium sulfate, which is recovered as a by-product,” wrote Forward (1953).
“The new process can produce high-purity nickel, copper and cobalt at a cost appreciably lower than if conventional smelting and electrolytic refining were used.”
Fort Saskatchewan refinery
In January 1952, Chemico got the contract for the design and construction of a nickel refinery. Forward (1953) noted that the integration of an ammonia plant with the metal recovery plant would place Sherritt Gordon in a favourable position to produce chemicals in addition to the planned production of nickel, copper and cobalt.
“When the plant commences operation in 1954, Sherritt Gordon will not only become a producer of refined metals but will also be entering the field of chemical production. From the outset, ammonium sulfate will be one of the chief products of the plant, its value exceeding that of either copper or cobalt,” he wrote.
The site selected for the nickel refinery was an 800-acre parcel of land outside Fort Saskatchewan, Alberta, approximately 24 kilometres from Edmonton.
“The Alberta site was dictated by the requirement for natural gas as the feedstock for ammonia production, this being prior to the development of widespread pipeline networks,” wrote Kerfoot (1999). “A site on the Canadian National Railway was mandatory, because this was a condition under which the CNR had agreed to build the rail link to Lynn Lake. A further consideration was that the site should have good access to the Port of Vancouver to facilitate the export of the ammonium sulfate by-product for which at that time there was no local market.”
Construction work began on the refinery in May 1952. The railway reached the Lynn Lake mine at the end of October 1953, and ore production and concentrate shipment started on schedule in November.
According to Chalkley et al (2004), leaching of concentrate started on May 24, 1954. “By June 19, the leach circuit was filled and by July 15 feed liquor was available for the metal recovery section,” they wrote. “On July 21, 1954, the first nickel metal was produced and met specifications. The plant reached 90 per cent of design capacity by the end of 1954 and operated at design capacity during 1955.”
In 1957, Sherritt Gordon acquired the Chemico patents related to autoclave processes. “These patents covered inventions, which were made or developed during the period 1947 to 1956, leaving Sherritt as sole owner of jointly developed technologies,” wrote Chalkley et al (2004).
D. Maschmeyer and B. Benson (CIM Bulletin, September 1965) reported on the first 10 years of utilizing the ammonia leach process at the Fort Saskatchewan refinery. “A continuing development program, along with the expansion of certain facilities, has enabled the refinery to attain a production rate of 31,000,000 pounds of nickel per year by hydrogen reduction,” they wrote. “This represents an increase of approximately 85 per cent over the original design capacity of the plant.”
R. Stauffer and S. Lindsay (CIM Bulletin, June 1967) recorded that a supplementary acid leach circuit, installed in May 1962, had increased total cobalt production capacity to over 700,000 pounds per year.
Legacy
Kerfoot (1999) noted that much of Sherritt’s current metallurgical and product technology can be traced back to work done during the development of the ammonia leach process. “Pressure leaching of sulfide ores and concentrates, using continuous horizontal autoclaves, has provided the basis for a thriving pressure hydrometallurgical process licensing business which currently offers processes for treating nickel mattes and concentrates, zinc concentrates and refractory gold ores and concentrates,” he wrote. “By 1980, 40 per cent of free world refined nickel metal was produced by this technique.”
When the Lynn Lake mine was shut down in 1976, Sherritt was forced to look elsewhere for its main supply of feed, according to Chalkley et al (2004). “The refinery had been augmenting mine output by treating small amounts of mattes and secondary materials since the early 1960s. For the next 15 years, numerous types of feed were processed,” they wrote.
In 1991, Sherritt started obtaining feed as a mixed sulfide from the Pedro Sotto Alba plant in Moa, Cuba. The Moa Joint Venture, which was created in 1994, is 50:50 joint venture between Sherritt and General Nickel Company S.A. of Cuba. “Today, Moa sulfides account for 95 per cent of the refinery feed,” noted Chalkley et al (2004).
On Feb. 23, 2019, the Fort Saskatchewan refinery produced its three billionth pound of finished nickel.